What Are Two Subatomic Particles Found in the Nucleus
It does not contain electrons. In fact the mass number of an element is the sum of its protons and neutrons.

Subatomic Particles You Should Know Atom Hubble Pictures Ancient
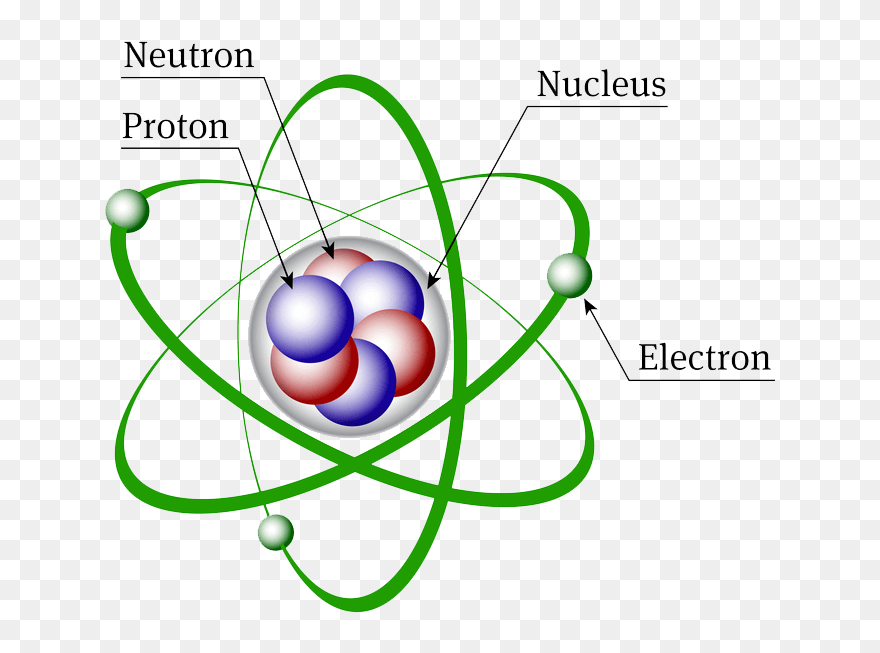
The nucleus contains two types of subatomic particles protons and neutrons.

. Click to see full answer. What do all isotopes of hydrogen contain. Two subatomic particles in the nucleus of any atom are protons and neutrons except hydrogen atom with only 1 proton no neutron.
Protons are particles with a positive charge while neutrons have no charge. The correct option is c. Identify the subatomic particles that are found within the nucleus of the atom.
The number of protons in an atom is equal to the number of electrons in it. Electron e is negatively charged particle that can occupy a volume of space orbital around an atomic nucleus. They each have one single proton Z 1 but differ in the number of their neutrons.
A new compound is formed. What two subatomic particles make up. A new element has been formed.
Electrons are found in a cloud floating around the atom. The nucleus only contains protons and neutrons. Electron and neutron D.
Protons are positively charged subatomic particles. The protons have a positive electrical charge and the neutrons have no electrical charge. What two subatomic particles are found inside the nucleus of an atom.
Which subatomic particles contribute the most to the mass an atom. There are equal numbers of protons and electrons in the atom. The nucleus contains two types of subatomic particles protons and neutrons.
Some important points regarding the discovery and properties of protons are listed below. A third type of subatomic particle electrons move around the nucleus. Protons have an atomic mass of 1 atomic mass unitAMU.
A third type of subatomic particle electrons move around the nucleus. Proton and neutron Protons and neutrons are found in the nucleus of an atom. A change has occurred in a nucleus.
It is not balanced. Since protons have a positive charge and neutrons are neutral the nucleus of an atom is electrically positive. The subatomic particles of protons and neutrons are found in the nucleus of an atom.
Protons and neutrons are the two subatomic particles located in the nucleus of an atom. Neutrons do not contain an electric charge. Two subatomic particles in the nucleus of any atom are protons and neutrons except hydrogen atom with only 1 proton no neutron.
The protons have a positive electrical charge and the neutrons have no electrical charge. The nucleus contains two types of subatomic particles protons and neutrons. What are the properties of subatomic particles.
Every atom has a specific set of identical protons and identical neutrons. Protons and Neutrons are two subatomic particles located in the nucleus of an atom. Protons neutrons and electrons B.
What subatomic particles are not found in the nucleus. Atoms have an equal number of protons and electrons. The nucleus contains two types of subatomic particles protons and neutrons.
Electrons which have a negative charge are particles that can found orbiting outside the nucleus of an atom. Which of the following are the subatomic particles found in the nucleus of an atom. Electron and proton 3.
Proton and neutron C. In the nucleus the two protons and two neutrons are depicted in red and blue. The nucleus of an atom is made up of two subatomic particles.
Which of the subatomic particles are found on the outside of the. Hydrogen _____ Atoms always have one proton. Which two particles are the primary determinants of an elements atomic mass.
Protons locate in the tiny dense region at the centre of the atom. A protons B electrons C neutrons. Protons and Neutrons together make up the nucleus of an atom and are hence called nucleons.
They make up a majority of the mass of an atom. Group of answer choices Other questions on the subject. Protons and neutrons are found in the nucleus of an atom.
Protons and electrons D. Protons and neutrons 4. In fact the mass number of an element is the sum of its protons and neutrons.
Subatomic particles called_____can be found at various distances from the nucleus. This depiction shows the particles as separate whereas in an actual helium atom the protons are superimposed in space and most likely found at the very center of. They make up a majority of the mass of an atom.
The nucleus of an atom is made up of two subatomic particles. The proton and the neutron. The protons have a positive electrical charge and the neutrons.
The proton and the neutron. Proton p is positively charged particle of the atomic nucleus. Since protons have a positive charge and neutrons are neutral the nucleus of an atom is electrically positive.
The protons have a positive electrical charge and the neutrons have no electrical charge. They are collectively called nucleons. Protons are positively charged particles.
Rutherford discovered this in his gold foil. Protons and neutrons are the two particles found inside the nucleus.
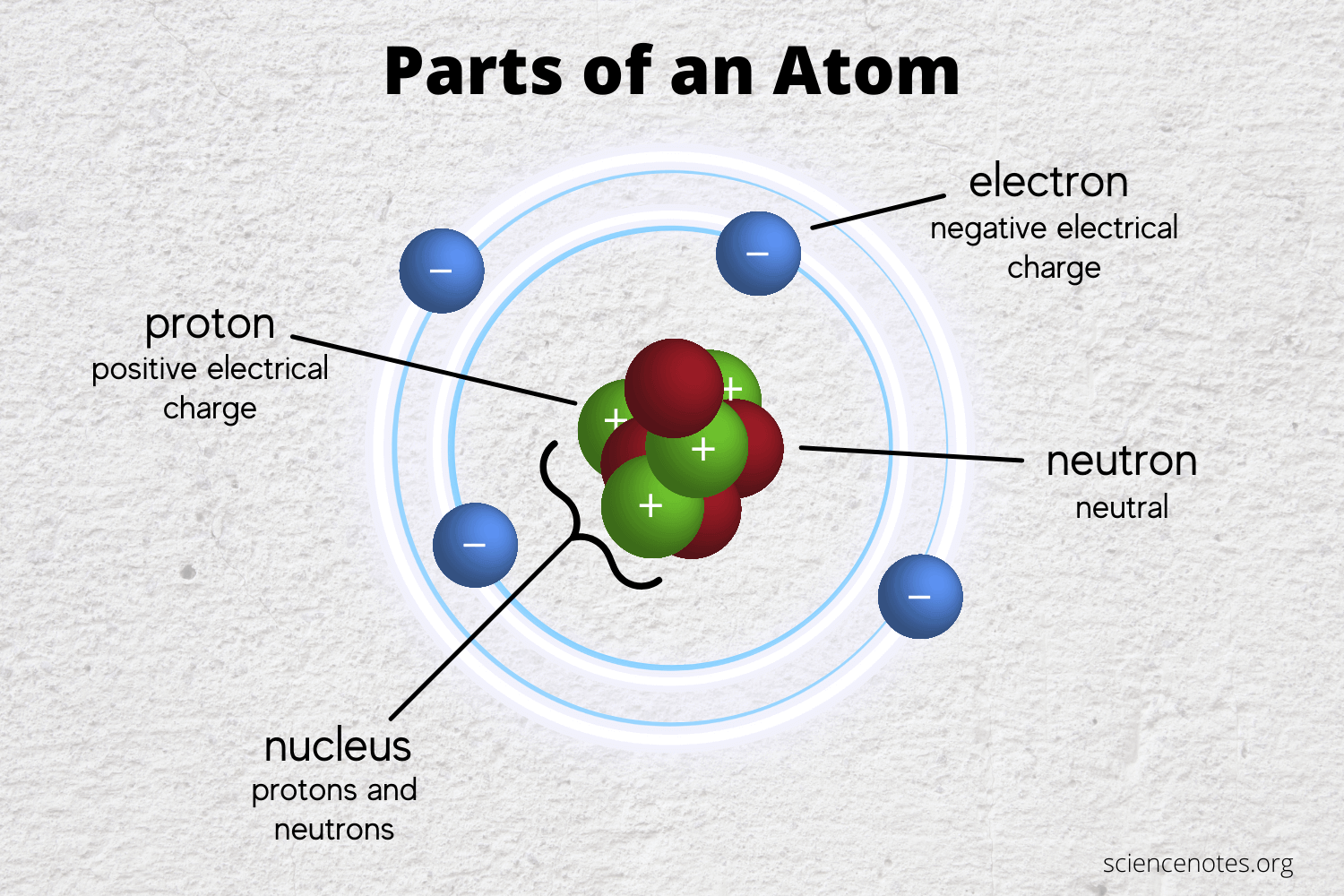
The Famous Types Of Subatomic Particles Praxilabs

Subatomic Particles Universe Today
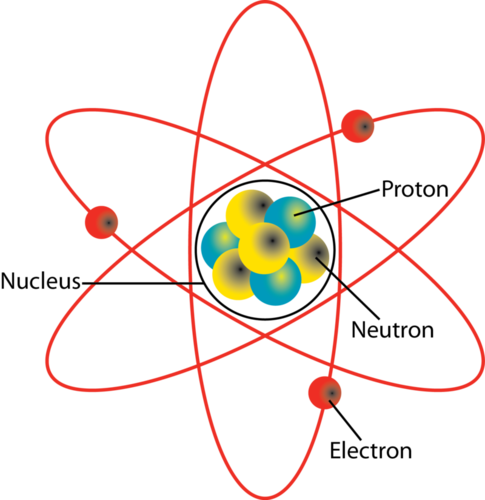
What Two Particles Are Found In The Nucleus Of An Atom Socratic

1 Constituents Of The Atom Google Search Atom Activities Atom Electron Configuration

Alpha Particle Easy Science Teaching Chemistry Particles

Pin On Deconstructing The Atom

Chemistry Task Cards 1 Atomic Structure Reactivity Properties Of Elements Middle School Science Activities Middle School Science Task Cards
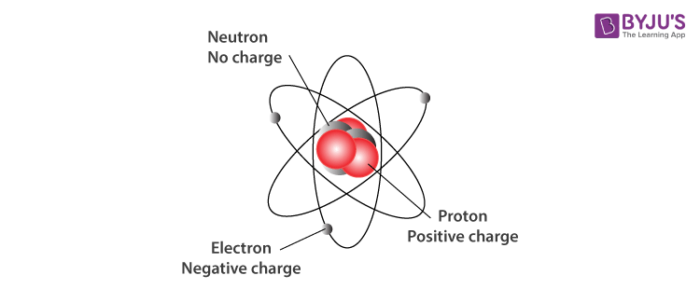
Subatomic Particles Definition Discovery And Key Features
Simplychemistry C1 1 2 What Is An Atom Sub Atomic Particles

Which Two Subatomic Particles Are Located In The Nucleus Of An Atom Brainly Com

Atoms And Subatomic Particles Exit Ticket Assessment Exit Tickets Atom Particles

Physicists Discover New Subatomic Particle Physics World Theoretical Physics Physics

Atomic Structure Understanding Subatomic Particles Printable Teaching Resources Education Lesson Plans Middle School Lessons

Visualizing The Innards Of Subatomic Particles Scientific American Blog Network Theoretical Physics Quantum Physics Quantum Foam
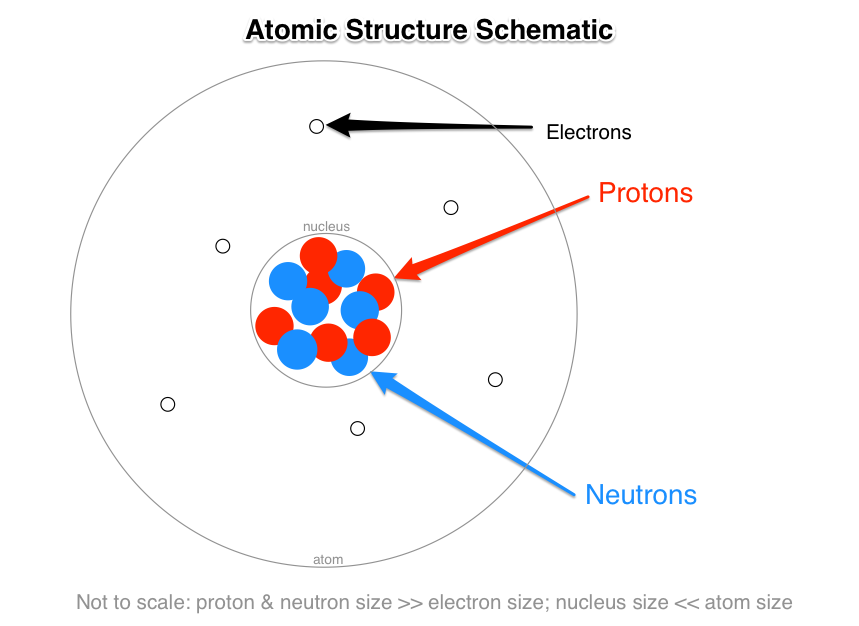
What Are The Names Charges And Locations Of The Three Types Of Subatomic Particles That Make Up An Atom Socratic


Comments
Post a Comment